Gadolinium is a rare-earth metal commonly used in important applications like magnetic resonance imaging techniques and jewelry manufacture. It is commonly represented as Gd and has an atomic number 64. Since its discovery in 1880 by Jean Charles Galissard de Marignac, it wasn’t available in its pure form until Paul-Emile Lecoq de Boisbaudran — a French chemist — carried out the first isolation in 1886. The Gadolinium element is actually named after a Finnish scientist called Johan Gadolin.
Further Reading: Seventeen Rare Earth Elements Introduction
Gadolinium is a highly reactive metal. Consequently, it is hardly naturally occurring but is found as a component of other minerals. Some minerals that contain gadolinium include monazite, gadolinite, and bastnasite. Almost 400 tonnes of gadolinium are produced in the world each year. It is usually found in Brazil, China, the US, Sri Lanka, and Australia.
Gadolinium has six naturally occurring isotopes and one radioisotope. The naturally occurring isotopes have a mass number of 154, 155, 156, 157, 158, and 160, respectively. The radio isotope’s mass number is 152. The most abundant isotope is 158Gd, while the least abundant isotope is 152Gd.

Properties
On the periodic table, gadolinium belongs to period six, and its block is the f-block. Its periodicity is responsible for most of its properties. Its outermost shell has two electrons, and its standard atomic weight is 157.25.
Gadolinium has a silvery appearance together with a hexagonal close-packed crystal structure. At standard temperature and pressure, this metal is solid. Its boiling point is 3273 K, while its melting point is 1585 K. Gadolinium’s most common oxidation state is +3, as is the case with most rare-earth metals.
Magnetic fields alter the temperature of gadolinium. Its temperature rises in the presence of a magnetic field but falls in the absence of a magnetic field. In addition, this rare-earth metal has magnetic properties. Below 293 K, gadolinium is ferromagnetic. However, above this temperature, it is paramagnetic.
Gadolinium is a ductile and malleable metal with varying density states. When gadolinium is solid, its density is 7.90 g/cm3. However, in a liquid state, its density changes to 7.4 g/cm3.
Gadolinium forms a black coating when it is oxidized in moist air. It reacts with dilute sulphuric acid and halogens like fluorine, chlorine, and iodine. In addition, it reacts with water to form gadolinium hydroxide.
The properties of gadolinium are similar to that of other rare-earth elements. Most of these properties result from its position on the periodic table. The properties of gadolinium determine how this rare-earth element is applied in various industries.
Applications
Gadolinium has a wide range of applications. However, it is not usually used on a large scale because of how rare it is.
Magnetic Resonance Imaging
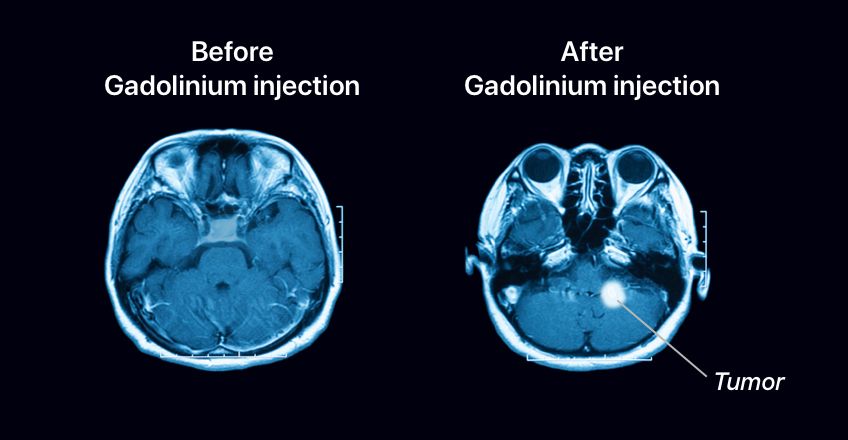
Gadolinium is well appreciated in magnetic resonance imaging. It is used to make internal organs and structures more apparent during magnetic resonance imaging. Substances that perform this function are called MRI contrast agents. It is usually let into the body intravenously. For gadolinium to be let into the body without causing any harm, it must undergo a process known as chelation. About a third of all MRIs use gadolinium as a contrast agent. It is used to detect conditions such as kidney inflammation, brain injury, cancer, and spinal cord injury.
Nuclear Reactors
Gadolinium is used in the manufacture of control rods that shield nuclear reactors. This is because gadolinium can absorb neutrons. This means it has a high nuclear capture cross-section. Absorbing these neutrons stops them from causing the fission of nuclear fuel.
Thin-Film Coating
Gadolinium sputtering targets and Gadolinium evaporation materials are used in deposition processes including semiconductor deposition, chemical vapor deposition (CVD) and physical vapor deposition (PVD).
X-Ray
Gadolinium is a phosphor. This means it is luminescent when it comes in contact with radiation. Thus, it is used in x-rays. X-rays are applied in medicine, aviation, and mining. In medicine, they are used to inspect internal body structures and organs to detect anomalies. In aviation, x-rays are used to inspect luggage in the airport. In mining, x-rays are used to discover and distinguish minerals.
Television
Amongst its other uses, gadolinium is used in making color television. This is possible because gadolinium is a phosphor.
Jewelry
A compound of gadolinium, gadolinium gallium garnet, can be used to make fake diamonds. This is used in the jeweling industry to create designs at a cheaper cost.
Alloys
Gadolinium is usually added to other elements to form alloys. When gadolinium is added to an element, it usually makes the element more resistant to corrosion and high temperature. Elements mixed with even as little as 1% of gadolinium show marked improvement compared to those containing no gadolinium.
Dopant
Gadolinium is used as a dopant. An example of a product created by using gadolinium as a dopant is gadolinium-doped ceria. This compound is known to have a high ionic conductivity. It is often used in oxygen sensors and solid oxide fuel cells.
Conclusion
Gadolinium is an important rare-earth metal. Its relative scarcity in the earth’s crust does not hinder it from being an asset in various industries. If you want to know more about rare earth elements, we would like to advise you to visit Stanford Advanced Materials (SAM) for more information.




