Introduction
Hydrogen is widely viewed as a promising source of green energy because when it burns or is used to produce power, it gives off only water. Many people believe that the way we produce hydrogen is just as important as the fact that it does not emit carbon dioxide. Today, one of the best ways to produce carbon-free hydrogen is through a process called electrolysis—using electricity to split water molecules into hydrogen and oxygen. This process can be very clean if the electricity comes from a renewable source.

Electrolysis and Polymer Electrolyte Membrane Electrolyzers
Electrolysis takes place in a device known as an electrolyzer. In these devices, there are two main parts—the anode and the cathode—separated by an electrolyte. A special type called the polymer electrolyte membrane electrolyzer has become very popular. These devices, sometimes called proton exchange membrane electrolyzers, work quickly, generate lots of power, and produce very pure hydrogen. Their compact design and high efficiency make them a favorite among those who want to use renewable energy for hydrogen production.
However, the high upfront cost of these systems remains a concern. The reason for the large expense is the need for rare and expensive precious metals. These metals are used as thin layers on certain parts of the device in order to get the best performance possible out of every unit.
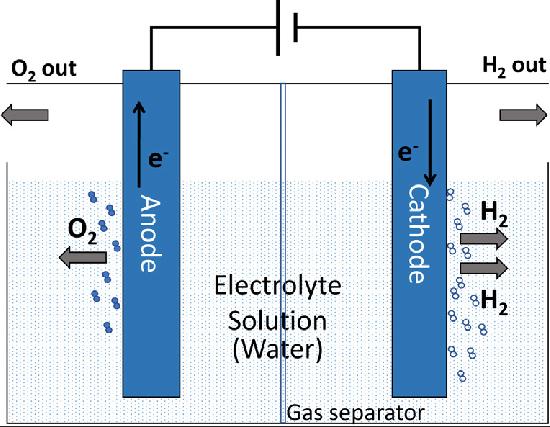
Challenges in Using Precious Metals
In a polymer electrolyte membrane electrolyzer, some parts receive special attention. For example, the bipolar plate, which helps conduct electricity and separate gases, is often covered with metals like platinum or gold. Similarly, the part that helps transport fluids, known as the porous transport layer, is also coated with platinum. These precious metals do not only boost the flow of electricity; they also protect the device from corrosion and help prevent a problem known as hydrogen embrittlement, which can weaken the system over time.
Even though these metal coatings work very well, they represent a large part of the overall cost. This is why the metals are applied in very thin films that use as little material as possible while still creating a reliable benefit. The process used to deposit these coatings is known as physical vapor deposition. It requires careful control of conditions in order to achieve a uniform layer on parts such as the bipolar plate and porous transport layer.
Physical Vapor Deposition Coatings: How They Help
Physical vapor deposition is a process in which metal is vaporized in a vacuum and then deposited onto a component in thin, even layers. This method has been used for many years in various industries due to its ability to produce coatings that are both robust and efficient. With proper use, it can improve the electrical conductivity of the device and extend the life of the electrolyzer.
When thin layers of precious metal are applied correctly, the overall system benefits in several ways. First, the electrical conductivity is improved. The efficiency with which electricity is used in the electrolysis process goes up. Second, the coating helps protect the underlying material from corrosion that can occur during operation. Protection against corrosion means that there is less need for repairs over time, further helping to reduce lifetime costs. Lastly, the metal layer minimizes issues like hydrogen embrittlement, keeping the device working smoothly even over long operating periods.
Economic and Operational Benefits
The savings from using physical vapor deposition coatings extend beyond the initial operational improvements. By applying a very thin yet effective layer of precious metals, manufacturers can avoid the need to use large amounts of these expensive substances. This approach lowers the overall cost of owning and running the electrolyzer.
In everyday practice, these enhancements mean that producers of hydrogen have a much more reliable system that operates efficiently under continuous use. By cutting down on the amount of metal required, the system not only becomes less expensive to build but also benefits from longer lifespans. This improved durability translates to lower maintenance costs and fewer interruptions in hydrogen production.
In several factories and pilot projects, operators have noted that systems using advanced physical vapor deposition coatings show less wear and a better performance profile compared to older models. This real-world feedback underscores the importance of such coatings in high-volume hydrogen production. When operators can depend on components staying in good working order, they can plan for long-term use with confidence.
Stanford Advanced Materials (SAM) and Their Role
In recent developments, Stanford Advanced Materials (SAM) has stepped forward as a reliable supplier of high-quality precious metal targets needed for the physical vapor deposition process. With decades of experience in materials science, SAM is well known for its commitment to quality and precision. Their tools are designed to coat electrolyzer components with the required thin layers that enhance efficiency and extend operational life.
SAM’s approach involves not only state-of-the-art equipment but also a deep understanding of the challenges that come with high-volume production. Their supply chain and machining expertise allow them to manufacture backing tubes and other components that meet very strict tolerances. This ensures that every component performs as expected during operation. Moreover, by recycling spent targets and used backing tubes, SAM keeps costs under control and promotes better use of precious metals.
Conclusion
Using advanced physical vapor deposition coatings is a practical way to improve the performance and durability of polymer electrolyte membrane electrolyzers. By applying very thin layers of precious metals, these systems benefit from better electrical conductivity, superior protection against corrosion, and minimized risk from hydrogen embrittlement. This method also helps manufacturers reduce costs over time, as less metal is needed without sacrificing performance.
The experience of many in the energy production field shows that these coatings work well when handled with care and proper technique. The combination of reliable coating methods and careful material selection makes a strong case for such an approach in hydrogen production. Stanford Advanced Materials (SAM) provides the high-quality supplies and expertise needed to implement these solutions with confidence.
For anyone looking to improve the efficiency and lifespan of their polymer electrolyte membrane electrolyzers, turning to advanced physical vapor deposition coatings is a wise choice. With the support of trusted materials suppliers like Stanford Advanced Materials (SAM), operators can look forward to a future where hydrogen production is not only clean but also cost-effective and reliable.



